- U-Report
VIVA – The United States Food and Drug Administration (FDA) has approved the first pill manufactured from human poop, the agency announced on Wednesday. It is the second human poop-derived treatment ever approved; the first was an enema-based treatment cleared for use in December 2022.
Previously, such "fecal microbiota transplants" were considered investigational treatments and were, therefore, harder for patients to access and often not covered by insurance.
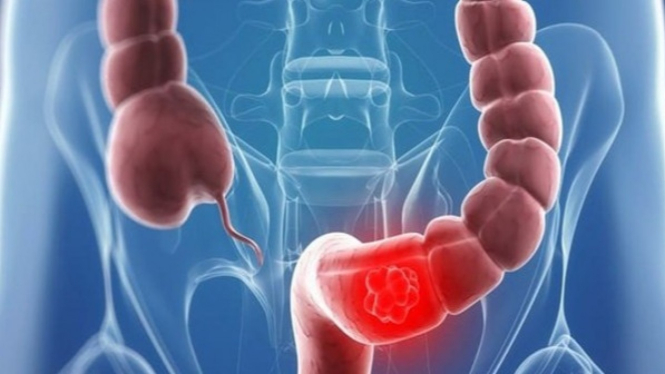
Like the approved enema treatment, the newly approved pill, called Vowst, also contains live bacteria and has been approved for use in people ages 18 and older as a preventive treatment for recurrent infections with the bacterium Clostridioides difficile.
Called C. diff for short, this infection is often acquired in healthcare settings after patients have taken antibiotics for a different infection.
Ilustrasi obat/vitamin.
- Freepik
Antibiotics can disrupt the balance of bacteria that normally populate the gut, and this gives C. diff the opportunity to proliferate.
The rapidly replicating bacteria secrete toxins that can lead to diarrhea, abdominal pain, fever, colitis (inflammation of the colon), and, in some cases, organ failure and death. C. diff infections are associated with about 15,000 to 30,000 deaths a year in the United states according to the FDA.
Those who recover from C. diff have a roughly 1 in 6 chance of developing the infection again within two to eight weeks of recovery, according to the Centers for Disease Control and Prevention.
The risk of these recurrent infections increases each time a person gets C. diff, in part because the antibiotics used to treat them further disrupt the gut microbiome, the community of microorganisms in the lower digestive tract.
So-called fecal microbiota products, made from healthy human gut bacteria, offer a new way to prevent recurrent C. diff by essentially replenishing the gut microbiome. And now, with the approval of Vowst, there's a version of the treatment that can be taken orally, rather than being administered as a liquid treatment into a patient's rectum.
"The availability of a fecal microbiota product that can be taken orally is a significant step forward in advancing patient care and accessibility for individuals who have experienced this disease that can be potentially life-threatening," Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in the agency's statement.
The Vowst treatment regimen involves taking four capsules once a day for three days in a row; patients start taking the drug two to four days after finishing a course of antibiotics for C. diff.
The donated feces used to make the pills is carefully screened for transmissible pathogens before being used in manufacturing, but taking Vowst still carries some risk of being exposed to pathogens, as well as to food allergens, the FDA cautioned.